The Collapsing Narrative
Posted by Andrew | Filed under A Farewell to Authority, Breakfast in the Ruins, Commentary, Environment, General, Gripes, Humour, The Destruction of History
A sign of our times is the constant harping on by the legacy media about things which are not really happening, and these are simply distractions.
The bizarre public conversation regarding apparently rising levels of carbon dioxide in the Earth’s atmosphere is a Will o’the Wisp, a phantasm. Common sense would suggest the opposite over geological time scales – that the combination of weathering and biological processes, which (as far as we know) are absent on other rocky planets, will eventually sequester all of the atmospheric carbon, at which point, plant growth will cease, and we will all starve to death.
If you pushed me, I would have to admit that there is so much Clown World activity these days that I often just sit back and laugh. This “climate science” farce, in which it is claimed that Armageddon is only years away, primarily due to the combination of burning fossil fuels and cow farts, clearly not only doesn’t coincide with observable facts, but is also hiding something that nobody ever seems to talk about. Now, it does seem strange to me that prestigious researchers don’t mention this, but it also shows that maybe well-educated and experienced people who should know better also don’t see it, even though it is right under their noses, and we might describe it as being part of “Chemistry 101”. Or maybe people are afraid to state the glaringly obvious?
What I am talking about here is the tendency of chemical reactions to proceed until they are no longer thermodynamically possible. Admittedly, this is somewhat difficult to illustrate and may seem somewhat obscure to many onlookers, but bear with me; this is real science, not journalistic gobbledegook. Remember: this is an experienced and world-published chemist and biochemistry graduate talking here, not the kind of “scientist” bemoaned by Thomas S. Kuhn back in 1962 (the year that I was born!) who spends his or her whole damned career trying to “verify” the theories of others rather than challenging them. Question everything.
Back to the chemistry…
Another thing we have to remember is that, for precisely this reason, Earth’s current conditions are nowhere near resembling what they were like when this planet was first formed. As we are talking primarily about the atmosphere and climate here, we should remember that, according to data from geological sciences, the atmosphere of this planet was originally unbreathable; it was toxic and contained components such as methane, ammonia and hydrogen sulphide, and it remained this way for millions of years because the dominant early lifeforms of the time – bacteria and their allies – produced these as the wastes from their respiratory processes.
All of that started to change when photosynthesis arrived, and a new waste – oxygen – started to be produced in vast quantities. The result of this was that the former kings of this domain – anaerobic bacteria – could not survive with oxygen diffusing into the waters, and they were forced to survive in places occupying the lowest positions in the oxygen gradient. Think of the dark, anoxic substrata of estuarine mud flats (and I know, because I went there sampling anaerobes when I was a biology student), and you will get the idea.
A big part of our current situation, then, is apparently due to an accident of nature – a transition from purely anaerobic chemical life processes to aerobic ones in geologically ancient and remote times, and resulting from the appearance of photosynthesis and the subsequent change of major atmospheric constituents from gaseous wastes such as methane, ammonia and hydrogen sulphide to gaseous oxygen, which is factually toxic to the surviving anaerobes.
We could illustrate this by referring to other rocky bodies in the Solar System to see how it might have been otherwise. On the one hand, we have Titan, the largest moon of Saturn, a frigid world with a thick, orange atmosphere composed largely of nitrogen and methane; on the other, we have a place like Mars, where the atmosphere is composed mainly of carbon dioxide, plus a few odd little components (such as methane). We are sometimes told in the popular scientific press that the former represents a primordial Earth, and from our discussion above, this seems to be true; from it we could conclude that the big difference is that the emergence of life made it more chemically dynamic. The same could be said of Mars: perhaps, if there were photosynthetic life there, the atmosphere would be strikingly different – much more like that of Earth is nowadays. It would still be quite thin, however, because of the relatively low gravity of Mars (compared with that of Earth, for example) – a factor overcome to some extent, in the case of Titan, by the frigid temperatures.
What I would suggest here is that the presence of life on Earth, and the dynamism it contributes to geological and atmospheric processes, has an additional effect: it slowly leads to the depletion of carbon dioxide by sequestering into other forms. For example, as carbon dioxide is soluble in water (a polar liquid in which it forms soluble carbonate anions, which can form solids with e.g. dissolved metal ions such as calcium, magnesium and copper), which is perhaps its most important characteristic from a purely chemical point of view, it is more immediately able to undergo reactions which can convert it into inaccessible forms; think of corals, for example. We won’t go into a discussion of the enzymes involved here, but simply remind ourselves that stony corals are “stony” because their polyps take carbon dioxide from the air (dissolved in seawater) and convert it into an insoluble carbonate. Some of this may return to the atmosphere when the polyps die and their calcareous skeletons begin to degrade, but if these are subsequently buried, the degradation would be prevented and eventually, the skeletons would become fossils in a rocky matrix, at least if the conventional process of fossilisation is correct. Since the primary source of carbonate for corals comes in the form of carbon dioxide dissolved in sea water, the long-term result of this would be the depletion of atmospheric CO2.
We might also remember that the shells of molluscs and many marine algae, both geologically ancient and modern, are likewise composed of calcium carbonate (aragonite), sequestered biologically and presumably only slowly weathered away when their owner dies. Again, there are plenty of fossils of these creatures and again, once encased in a rocky matrix, the material is sequestered and chemically inaccessible. Think of the huge deposits of ancient microscopic marine algae such as we find on the south coast of England – and how many such deposits are not exposed to weathering due to still being buried deep under subsequent rock strata. They might not even go this far – if buried in mud, perhaps no further reactions are possible with these materials (concrete, anyone?).
An additional material for sequestering could be wood. Non-woody plants fix carbon into sugars via glycolate (mainly), and the plants may then transform it into sugars or oils. The sugars are partly stored and partly used for structural purposes – polymerised into amylose (starch) for future energy usage, or further polymerised into cellulose to create wood fibres. There would be an annual carbon dioxide flux according to how many non-woody plants die and decay, but less so in the case of woody plants, especially in the case of large trees in (for example) Earth’s extensive boreal and antiboreal forests. The boreal forests might be interesting here on account of their evergreen content – resinous pine needles again sequester carbon and rot away very slowly, unlike the leaves of deciduous trees. You do not see processes like these on Titan or Mars (or even on Venus).
Venus is interesting because it has an atmosphere composed mainly of carbon dioxide [1] plus a lesser amount of nitrogen and sulphuric acid; it is also much denser than the atmospheres of the other rocky bodies. Is Venus so hot because of the carbon dioxide? Perhaps the truth is that Venus is a relatively recently-formed planet (according to thousands of stories in global folklore; check out the works of Immanuel Velikovsky for more information), and what we are seeing is the remanent heat of its formation, which probably is being lost only slowly because of its closer proximity to the sun.
Our main point, however, even in the case of Venus, is that the one thing not present is life; the atmospheres of these other rocky bodies, according to conventional wisdom, represent possible primordial states from which our current atmosphere could have developed – if life were present. Left alone, the existing geological and atmospheric processes there would presumably stay the same, forever. On Earth, however, the geological/geochemical record, as it is currently understood, seems to indicate three basic phases: the initial, lifeless and anoxic, primitive post-formation atmosphere; a second phase, resulting from chemical life processes, and still anoxic and too toxic for modern-day life; and finally the almost-end stage which we have today, caused by plant photosynthesis and the global availability of oxygen, which is itself toxic for surviving anaerobes. However, if our hypothesis here of time-dependent CO2 depletion is correct (and it should be because it is thermodynamic at its heart), we are living in the end stages of survivability on this planet not because of pollution, but simply because the chemistry of carbon dioxide allows it to dissolve easily in water, which is where it becomes available to biological processes, either within the watery photosynthetic tissues of plants, or by being absorbable into animal tissues, where enzymes can transform it into a solid.
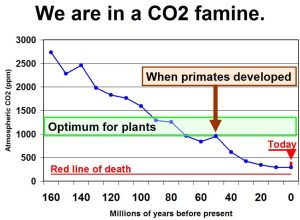
Our eventual fate, then, is starvation, as the levels of available carbon dioxide in the atmosphere decline past the point at which plant life can convert it into sugars and oils. If it falls to half its current level, plant life will start to die, and as animals depend upon plants to maintain the food chains, they also will become extinct – including ourselves. The end-point of Earth’s development is starvation of its inhabitants due to the irreversible mass sequestration of the original atmospheric carbon dioxide. We should note that during periods when the CO2 levels rise, plant life flourishes; we should also remember that when growers of crops, using greenhouses, want to enhance growth, they add CO2 to the closed atmosphere in which they grow the plants. You can visualise current CO2 levels compared to those of the geological past in the graphic, “We are in a CO2 famine”, in a previous entry here.
What stimulated me into writing all of this (and it took a few days of cogitation for it to all crystallise in my mind) was an article at ZeroHedge by our friend, “Tyler Durden” [2]. According to an article in The Washington Post (inaccessible to we plebs due to their paywall, but reported on X as well), the corrected geological record for the past 500 million years shows unequivocally that mean global temperatures are in steep decline and have been for the last 50 million years. Essentially, temperature measurements have been compromised for a long time by the change of local temperatures resulting from increasing urbanisation, so that formerly isolated measurement stations (which used to be in the countryside) show incorrect temperatures resulting from being caught up in the heat bubbles surrounding cities:
“WaPo journalists cited a new study about Earth’s global surface temperatures over the last 485 million years. In 2023, Earth’s average temperature reached 58.96 F (14.98 C), well below the average 96.8 degrees F (36 degrees Celsius) the study showed around 100 million years ago. The trend shows Earth’s temperatures have been sliding for 50 million years. ” [2]
The interesting thing about this is that, if CO2 is an effective “greenhouse gas” (and it is often said that water vapour is more effective, not least because the cloud cover on Earth represents a self-correcting system; ditto for methane), it would actually make sense that mean global temperatures should decline in tandem with declining CO2. However, we might then have to ask a question like: “… but the atmosphere of Mars is almost entirely made up of carbon dioxide, and it’s freezing there!”, and according to authorities such as NASA [3], a good day on Mars would be barely temperate; nights there would put Antarctica to shame. Clearly, the whole “Global Warming” hypothesis is unsupported by any available evidence.
Personally, as I suggested earlier, I tend to find all of this amusing, but it is for precisely this kind of reason that I also gave up on a career in science long ago; I even left the UK and changed careers because after graduating, it proved impossible to get a post in my chosen area of study, and you can’t live on air and promises. Essentially, however, there seems to be no evidence whatsoever that establishes a causative link between increasing CO2 levels in Earth’s air and rising atmospheric temperatures; what evidence there is indicates that it has been declining over geological time, and that on the same timescale, global atmospheric temperatures have likewise been falling, and that this decline continues despite widespread deforestation and the burning of fossil fuels which are alleged to have the opposite effect. Whole spurious areas of pseudoscience and lucrative careers have been built upon this foundation of sand.
Remember, folks: all of this is just gaslighting and it isn’t really happening. Nothing in the foregoing discussion needed any great leaps of logic or mathematical analysis; all of that has already been performed at various locations in academia. We have merely linked together a few salient points, most of which were apparently produced by that same collective academia long ago.
A final caveat is as follows: we only know what we can see right now. We can’t jump into a time machine, like Doctor Who’s TARDIS, and go on a jolly romp through time seeing exactly what happened in the remote past. Our experience of time is purely one-dimensional: we have no idea how this planet formed, how many planets there were originally in total, what happened to them or even how they were arranged around the sun; take the Electric Universe theory seriously (and indeed, I do), and the first thing you realise is that we don’t even know whether the planets that we see today even belong to this one sun, or whether they wandered or were snatched in from elsewhere; the surprising heterogeneity of the visible planets is very suggestive of this. If the EU adherents’ accounts (based upon legends and traditional stories handed down from those who were there) are anything to go by, both our primary (sun) and the arrangement of the original planets were probably very different. We have to tread carefully because we are at the end of a long set of processes, plus we have to be careful when we try to apply analogies from our observation, as the analogies may be incorrect.
Real science is a pursuit in which a hypothesis must be falsifiable in order to be supported, at least until it is either fully or partially disproven by new research or evidence; everything in science is therefore purely provisional. Only a nitwit politician can stand up and assert without evidence that “the science is settled”. Science is never settled; it’s a cat on a hot tin roof, and a healthy science is one in which different hypotheses compete to see which one(s) better represent(s) reality. When only one hypothesis is presented and, as so often in this particular case, others are deliberately excluded from public discussion or subjected to public ridicule, you know that somebody is up to no good. Two or more options are healthy competition; a single option is propaganda.
References:
[1] https://en.wikipedia.org/wiki/Atmosphere_of_Venus : “Venus’s atmosphere is composed of 96.5% carbon dioxide and 3.5% nitrogen, with other chemical compounds present only in trace amounts.[1] It is much denser and hotter than that of Earth; the temperature at the surface is 740 K (467 °C, 872 °F), and the pressure is 93 bar (1,350 psi), roughly the pressure found 900 m (3,000 ft) under water on Earth. The atmosphere of Venus supports decks of opaque clouds of sulfuric acid that cover the entire planet, preventing optical Earth-based and orbital observation of the surface.”
[3] https://science.nasa.gov/mars/facts/ : “The temperature on Mars can be as high as 70 degrees Fahrenheit (20 degrees Celsius) or as low as about -225 degrees Fahrenheit (-153 degrees Celsius). And because the atmosphere is so thin, heat from the Sun easily escapes this planet. If you were to stand on the surface of Mars on the equator at noon, it would feel like spring at your feet (75 degrees Fahrenheit or 24 degrees Celsius) and winter at your head (32 degrees Fahrenheit or 0 degrees Celsius).”
Tags: chemistry, farce, gaslighting, geochemistry, global warming
The CO2 famine
Posted by Andrew | Filed under A Farewell to Authority, Breakfast in the Ruins, Commentary, Environment, General, Gripes, The Destruction of History, Uncategorized
It’s quite incredible that, despite the use of different chemical proxies to determine the carbon dioxide in past eras which demonstrate that over vast amounts of time, atmospheric CO2 was vastly higher than it is now, there are people that insist that we need to not release it into the air.
Increasing the carbon dioxide in the air allows plant life to flourish; that’s why growers add it to the air in their greenhouses – with other conditions normal, higher carbon dioxide allows the deposition of more biomass.
However, this graphic suggests that we are in danger precisely because we listen to Chicken Little so much. Those California Redwoods weren’t made in a day, and they needed carbon dioxide. Maybe that’s why they took so long to grow!!!
I just saw this on Twitter… read it and weep:
Towards the Alternative Tech Life (I)
Posted by Andrew | Filed under A Farewell to Authority, Commentary, Computing, Environment, General, Health, The Destruction of History, Uncategorized
As regular readers may know, I have been trying (not altogether successfully, alas) to escape the demesne of the evil Bill G. and his hangers-on ever since leaving benighted Blighty. The most interesting aspect of this, right now, adventures with Linux aside, is what is happening to both what have come to be referred to as the “legacy media” and, indeed, to YouTube itself. Recent events and the behaviour of YT themselves have started to make it crystal clear what is happening, at the same time fuelling the rise of alternative media. It would all be so amusing if it were not so serious.
One thing which has emerged on the Internet since way-back-when is the desire of individuals to upload all kinds of goofy shit to the Internet. Originally there were few suitable outlets, so when YT came along, people naturally thought that this was an ideal place for their video material, and gradually said material became longer in duration and more intense (although not necessarily more “serious”) in terms of content. In recent years, however, we have been seeing that these outlets are in fact a form of social control, and the more serious (and relevant) content producers have been sidelined, banned and forced to resort to alternative means to get their message out. All I can say is that this is a very good trend, as YT itself seeks to become ever more irrelevant and to imitate the failing model of legacy media, which fewer and fewer seem to want to subscribe to because it no longer has content (or viewpoints) which relate to theirs.
Now I have subscribed to a lot of these alternative platforms over the last few years for one reason or another, the main reason being that, for whatever reason they may have, the legacy media are conspicuously controlled by a group (or groups) who clearly do not have the well-being of the various nations at heart: someone’s agenda is being played out and the media are suspiciously compliant and supportive of that agenda.
There have been many recent cases where serious incidents have occurred and the mainstream (“lamestream”) media have not reported them at all; it’s like an ongoing malaise which affects everyone, but especially the brain-dead normie types for whom media always report the truth and cannot be questioned. As an example, large-scale food repositories across the US have been (and continue to be) subject to sudden acts of what appear to be arson – suddenly and inexplicably catching fire, therefore depriving whole local populations downstream of food supplies.
The latest incarnation of this has been an outbreak of train crashes. You may perhaps have heard of the recent vinyl chloride shipment crashing in Ohio and the decision of the local authorities to actually set light to the shipment, ostensibly to prevent the containers from exploding, but little is being said about the disastrous effects of this not only to the locals and their wildlife and water courses, but also the eastward progression of the polluted air towards the Atlantic. Joker has recently put a video out about this and you should watch it:
While it remains the case that there is a lot of fine material still available on YT, it is increasingly a place where anyone who has a message in conflict with their policies can be demonetised and even de-platformed, for reasons which are often arbitrary and unavailable for public scrutiny; science there is often a domain dominated by virtue signallers who are merely acting as propagandists for some official paradigm, and who essentially present as brain-dead repeaters.
Against this backdrop, a whole range of new platforms have arisen to which creators on YT have increasingly been taking resort as it has become less possible to air their material and views there. I thoroughly recommend you to get away from YT and find out where so many well-established creators have set themselves up. You will not be disappointed!
Breakfast in the Ruins II
Posted by Andrew | Filed under Breakfast in the Ruins, Environment, Lost Geographies
Absolutely. I have nothing to add to this.
Why Bosses Are Bad…
Posted by Andrew | Filed under Environment
A great article which I came across by accident today:
http://www.killerculture.com/why-bosses-are-bad-and-other-problems-with-the-work-forced/
Definitely worth a read… it’s exactly what I have been thinking about work for ages.
Grouped under ‘Environment’, but maybe it should be under ‘Health”…
How We Should Be Making Our Own Food
Posted by Andrew | Filed under Environment
This is how it used to be back in England… people used to grow their own food a lot more, and there was food self-sufficiency. Funny how the West has this bizarre habit of decrying Russia when the Russians seem to get an awful lot of things right!
http://www.trueactivist.com/40-of-russias-food-is-grown-from-dacha-gardens/
The West has instead become dominated by large agribusiness concerns whose main aim seems to be to dominate us and force their disgusting products down our throats, whether we like it or not. When will we ever wake up?
Fukushima… Why No Reporting?
Posted by Andrew | Filed under Environment
Since the subsea earthquake in the Pacific and the devastation of the tsunami which followed, there has been a disturbing silence in the media. North America was downwind and there is a large plume of radioactive water now sloshing against the western seaboard; there are mass die-offs of animals and for some reason, when questioned by the media, no apparently reputable scientists will say anything other than: “We don’t know why; it’s a mystery.”
Really? Get a load of this video with Dr. Helen Caldicott, M.D. … she has some unfortunate socialist leanings but with my own background in biomedical sciences and as a published chemical analyst [1], I do not consider that sufficient reason to doubt her experience and opinions regarding the dangers of excessive environmental radiation.
Do take careful note of her comments about the activities of the Abe government in Japan in particular…
I am the author of:
Holmes, Andrew: Rapid Spot Testing of Metals, Alloys and Coatings. Finishing Publications Ltd., Stevenage, England (2002). ISBN 0-904477-25-8.
Holmes, Andrew (1999): More Spot Tests for the Metal Finisher. Metal Finishing (New York) [b]97[/b]:10, 10-15 (later translated and printed in [i]Galvanotechnik[/i] in Germany).
Holmes, Andrew (2000): A practical introduction to ion selective electrodes – part I: Theory. Metal Finishing (New York) [b]98[/b]:10, 18-23.
Holmes, Andrew (2000): A practical introduction to ion selective electrodes – part II: Methods. Metal Finishing (New York) [b]98[/b]:11, 38-45.
Edited: July 5th 2015